 | New Jersey Society of Interventional Pain PhysiciansAn Affiliate of the American Society of Interventional Pain Physicians |
in the news
New Jersey State Election Update
Laurie Clark – NJSIPP Legislative Counsel
Governor’s Race
On Tuesday, November 4, Congresswoman Mikie Sherrill was elected to be the 57th Governor of New Jersey. Top vote getters: The Governor – elect received 1, 829, 147 votes (56.5%) Jack Ciattarelli received 1, 392,136 votes (43.0%). The new Governor will be sworn in on January 20, 2026. We congratulate the new Governor-elect and look forward to working with the Governor and the new administration on critical healthcare issues.
Legislative Races
The New Jersey General Assembly will remain in Democratic control after Tuesday’s election. The members of the Assembly Democratic Caucus convened in Trenton yesterday to select the Leadership Team for the 222nd Legislative Session. Speaker Craig J. Coughlin was chosen to serve a historic fifth term as Speaker of the New Jersey General Assembly.
The Caucus met following Tuesday's election in which high turnout helped power Democratic victories across the state. Democrats in the Assembly successfully defended every seat and flipped an additional three seats for a 55 - 25 majority, with some races still not called. That is the largest majority held by Democrats in fifty years.
The gains in Tuesday's election show a continued voter shift to the Democratic Party. Since the 2023 election, Assembly Democrats picked up nine seats. The last time a party picked up four or more seats in consecutive elections was 1989-1991
The Caucus also voted to return Majority Leader Louis D. Greenwald (D-Camden, Burlington) to serve an eighth term in his leadership position. Speaker Pro Tempore Annette Quijano (D-Union) will begin her first full-term in this position, and Assemblywoman Linda Carter (D-Somerset, Union) will begin her first full term as Majority Conference Leader
Senate President Nick Scutari and Senate Majority Leader M. Teresa Ruiz were both unanimously reelected to their leadership positions for the 2026-27 legislative session by their Senate Democratic colleagues today.
Assemblyman John DiMaio was selected once again to be the Assembly Republican Leader.
Senator Anthony Bucco was re=elected as the Senate Republican Leader.
The members of the Senate Democratic Majority voted for the third time to have Senate President Scutari and Senate Majority Leader Ruiz continue in the top Senate posts they first assumed for the 2022-23 legislative session.
Reorganization of the 222nd Legislative Session will take place on January 13, 2026, when all members will be sworn in and an official vote on leadership will take place.
New Jersey Department of Banking and Insurance PIP Fee Schedule Revamp Looms
ICYMI – The NJSIPP Team was remarkably busy over the past few months. We responded to the proposal and met with key legislators. However, there is still a threat of this happening during the transition period between administrations.
We are continuing our work. Further updates will be forthcoming.
Senate Health Committee Considering Voluntary Non-Opioid Directives on November 13
The Legislature is back and the “Lame Duck Session” is officially underway. On Thursday, November 13, the committee has a full agenda including:
S4337/A5595 Allows individuals to establish voluntary nonopioid directives
Under the bill, an individual who is 18 years of age or older, an emancipated minor, or a patient’s authorized representative, as this term is defined in the bill, may execute a voluntary nonopioid directive stating that an opioid may not be administered or prescribed to the individual.
A voluntary nonopioid directive is to be made on a form that is to be developed by the Department of Health and published on the department’s Internet website. Nothing in the bill is to prevent an opioid from being prescribed to a patient if deemed medically necessary.
A health care professional, a health care facility, or an employee of a health care professional or health care facility is to be immune from disciplinary action by the Department of Health or a licensing agency or board for any act that was done to comply with the bill’s provisions. Further, a health care professional, a health care facility, or an employee of a health care professional or health care facility is to be immune from any civil or criminal liability for failure to administer, prescribe, or dispense an opioid, and for inadvertent administration of an opioid, to an individual who has a voluntary nonopioid directive, if the act or the failure was reasonable and done in good faith._____________________________________________________________________________________________________________________
NJSIPP Board Meeting with NJ Senate Leader Nick Scutari: Safeguarding Access to Care Amid PIP Fee Schedule Reform
October 15, 2025

Yesterday, the board of the New Jersey Society of Interventional Pain Physicians (NJSIPP) convened an important meeting with Senator Nick Scutari, Senate President and longtime champion of consumer protection and health care reform. The core focus: proposed changes to New Jersey’s Personal Injury Protection (PIP) medical fee schedule and ensuring that injured citizens retain access to high-quality care.
Key Highlights & Takeaways
1. Balancing cost control and patient access
Senator Scutari underscored the necessity of regulating medical reimbursement rates under PIP—but he also affirmed that any reform must avoid creating a “payment cliff” that would push providers to refuse PIP patients or refuse necessary services. The Senate leader emphasized that affordability for insurers cannot come at the expense of care providers’ viability or patient outcomes.
2. Protecting injured patients from balance billing
One of the strongest protections under discussion is a prohibition on balance billing for medical services tied to PIP claims. Under legislation such as S3963, unreimbursed medical expenses above PIP limits would fall under the same regulated fee schedule, and providers would be barred from demanding additional payment from patients.
3. Indexing and updating the schedule
We discussed a biennial inflation adjustment mechanism (e.g. cost-of-living, updates for new procedures) to keep the fee schedule responsive to real-world costs—so providers aren’t perpetually operating at a loss. Senator Scutari expressed openness to rule-making flexibility, especially for rural or specialty practices.
4. Stakeholder voices and transparency
NJSIPP representatives pressed for a transparent rule-making process, with sufficient public comment periods and input from providers, insurers, and advocacy groups. Senator Scutari pledged his support for an open, iterative approach rather than “last-minute” changes behind closed doors.
5. Ensuring continuity of care
Above all, the conversation returned again and again to the injured New Jerseyans who depend on PIP coverage to access rehabilitation, therapies, diagnostic imaging, and other critical services post-accident. It’s vital that reforms not inadvertently narrow provider networks or lead to care deserts for the most vulnerable.
✅ Why This Matters
- Patients: Without careful calibration, a lower reimbursement schedule may discourage participation from providers, limiting access for injured individuals.
- Providers & Clinics: Practices already operating on tight margins must absorb cost pressures; meaningful rate updates & inflation indexing are essential for sustainability.
- Insurers & Regulators: A transparent, predictable framework supports better rate-setting and risk management.
- Policymakers & Advocates: This board meeting is a model for constructive policymaking—bringing real-world professionals, legislators, and public-interest voices together before rules are finalized.
Call to Action:
- If you are a provider, insurer, patient advocate, or legislative staffer concerned about PIP reform in New Jersey, I encourage you to:
- Monitor upcoming rule-making hearings
- Submit comments or testimony during public notice periods
- Engage your State Senator and Assembly member
- Collaborate through NJSIPP or allied professional associations
- Let’s make sure PIP reform strengthens, rather than weakens, access to care in New Jersey
________________________________________________________________________________________________________
NJSIPP LEGISLATIVE UPDATE
July 1, 2025
Laurie Clark – Legislative Counsel
GOVERNOR MURPHY SIGNS FISCAL YEAR 2026 BUDGET INTO LAW
Ambulatory Surgery Center Tax Increase– Effective July 1
Despite our best efforts to stop it, the elimination of both the current $300,000 floor and the $350,000 CAP on the amount of the tax was passed in a standalone bill and signed into law on June 30. The new law is targeted to save $70 million over this fiscal year. Once the Legislature returns to session, coalition efforts will begin to attempt to change the assessment to net rather than gross receipts.
Known as the “Healthcare Finance Enhancement Act” (Assembly Bill 5809), the new law brings significant changes to the assessment of ambulatory care facilities, including ambulatory surgery centers, surgical practices, and licensed imaging centers (ACFs). Prior to the passage of the new law, ACFs were required to pay a 2.95% assessment on gross receipts over $300,000, with a cap on the assessment of $350,000. Under the new law, beginning July 1, 2025, the assessment is set at 2.5% of gross receipts, and both the floor of $300,000 and the cap of $350,000 have been removed. Further, while certain facilities, such as one (1) room surgical practices, were previously exempt from the assessment, these facilities will now be subject to the same assessment.
These changes will impact almost every ACF in New Jersey. While many facilities will have a reduced financial burden due to the decrease in the assessment percentage, many other facilities, such as surgical practices, will no longer be exempt from an assessment. Additionally, facilities that generate larger gross receipts will be assessed higher amounts, as the $350,000 cap has been lifted, and facilities that generate gross receipts under $300,000 will no longer be exempt.
The $58.78 billion Fiscal Year 2026 (FY2026) budget, which was passed by the Legislature and signed by the Governor, redirects over 75 percent of the total budget back into local communities in the form of grants-in-aid for property tax relief, social services, higher education, as well as State aid to schools, municipalities, and counties.
FY26 Budget Highlights
- Final FY26 Budget by the Numbers
-
- State appropriations: $58.78 billion
- State revenue: $57.31 billion
- Surplus: $6.7 billion
- Spending increase from FY25 to FY26: 4%
- Spending increase from budget proposed by Legislature in February to final budget in June: $767 million or 1.3%
- 5th straight year of 100% pension payment
The Legislature is expected to return to session in November.
________________________________________________________________________________________________________
UPDATE: GOVERNOR MURPHY’S NJ STATE FISCAL YEAR 2026 BUDGET
I attended in person, the Governor’s Budget Message and the Commissioner of Human Services, Medicaid Briefing.
For now, the proposed $58.1 billion budget is presented “status quo” without large programmatic cuts. However, the Governor did not rule out a “break the glass” emergency.
As you can see from the news coming out of Washington, DC, the House and US Senate passed different budget bills. There are also varying statements concerning the Medicaid/Medicare funding.
The Federal Medicaid funding is in danger, and everyone was encouraged to stay in contact with their representatives in Congress. New Jersey currently receives 50% federal match on Medicaid spending from the federal government. March 14 will be a pivotal day in Washington, DC as the final Congressional Resolution (CR) to fund the government as well as the debt ceiling must be re-authorized by Congress.
This state budget provides $6.3 billion for Medicaidmich for which we receive the same amount in federal matching funds. Currently, 1.8 million individuals utilize these programs.
Ambulatory Surgery Center Tax Changes
There are changes to the Ambulatory Surgery Center Tax that will negatively impact the owners of larger centers. Governor Murphy is proposing aligning the ASC tax rates with federal Medicaid Provider taxes allowing a reduction in the nominal rate from 2.95% to 2.5% but eliminating the cap. This is estimated to raise more than $60 million in new state revenues.
Primary Care Funding for Medicaid/Medicare Parity
An increase in current Medicaid Reimbursement for Primary Care. Including a new $49 million appropriation.
Attracting more OB-GYN’s
A new program to attract more OB-GYNs to New Jersey and retain the existing workforce is accomplished by increasing reimbursement rates.
Hospital Funding
Hospitals will receive $3.6 billion in direct state funding under this proposed budget. This represents $2.9 billion in increased funding since Governor Murphy took office.
Graduate Medical Education Funding
The proposed budget includes $336 million in funding including the supplemental and trauma components
This a partial list of some of the major healthcare components of the proposed budget. More will be provided in the next update. The Legislative review of the budget begins now. We will be fighting all elements of the budget that negatively impact on patient care….
Renew your membership and donate to PAC!
New Jersey State Legislative Update
Laurie Clark – Legislative Counsel to NJSIPP
February 13, 2025
We started the New Year with positive news on the legislative and regulatory fronts!
Telemedicine Parity Extension Signed into Law
Bill Was Signed on December 31
On December 19, both Houses of the Legislature voted to pass S2988/A3853 sponsored by Senator Vin Gopal and Assemblyman Herb Conaway.
This bill amends section 11 of P.L.2021, c.310 to extend the end date from December 31, 2024 to July 1, 2026 during which time a health benefits plan in this State is to provide coverage and payment for health care services delivered to a covered person through telemedicine or tele-health at a provider reimbursement rate that equals the provider reimbursement rate that is applicable, when the services are delivered through in-person contact and consultation in New Jersey, provided the services are otherwise covered by the health benefits plan when delivered through in-person contact and consultation in New Jersey.
The bills were signed into law on December 31. The law became effective immediately and extends the pay parity regulations for New Jersey Insurance Coverage until July 1, 2026.
Please note that Medicare Regulations on Telehealth Pay Parity have been extended until March of 2025. We await further Congressional action in the new session which begins tomorrow.
New Jersey Department of Banking and Insurance Issues Bulletin on Prior Authorization Law
On December 31, 2024, the New Jersey Department of Banking and Insurance issued several key bulletins effectuating the provisions of laws signed in the previous legislative session (2023). These Bulletins have the full force of law and are mandatory for compliance by insurance carriers operating in New Jersey beginning on January 1, 2025.
Please review the bulletin concerning prior authorization at the link below.
We are especially happy about the provisions that require a physician of the same specialty be directly involved with the internal appeal. Our society worked very hard on those provisions.
Personal Injury Protection Auto Insurance Reforms Underway
On Monday, the Senate Commerce Committee chaired by Senator Joseph Lagana reported favorably and with committee amendments Senate Bill No. 1473 (Lagana/Bramnick).
As amended, this bill revises the personal injury protection coverage for basic automobile insurance policies from $15,000 to $20,000 and requires a minimum personal injury protection coverage of $50,000 for standard automobile liability insurance policies. Under the bill, every basic automobile insurance policy will be required to provide personal injury protection coverage in an amount not to exceed $20,000 per person per accident, and every standard automobile liability insurance policy will be required to provide personal injury protection coverage that shall be no less than $50,000 per person per accident. Current law requires basic automobile insurance policies to provide personal injury protection coverage in an amount not to exceed $15,000 per person per accident, with no minimums required for standard automobile liability insurance policies. Additionally, the bill requires insurers to inform a named insured that policy limits for the insured’s standard automobile liability insurance policy or basic automobile insurance policy have increased during the renewal period of the policy but exempts insurers from needing a signed coverage selection form before increasing these limits.
This bill was pre-filed for introduction in the 2024-2025 session pending technical review. As reported, the bill includes the changes required by technical review, which has been performed.
COMMITTEE AMENDMENTS:
The committee has amended the bill to:
(1) revise personal injury protection coverages for basic automobile insurance policies from $50,000 to $20,000;
(2) require insurers to inform a named insured that policy limits have increased when the named insured has renewed a policy of insurance and exempt insurers from receiving a signed coverage selection form before increasing a policy’s limits;
(3) update current law reviewing personal injury protection options for standard automobile liability insurance policies;
(4) revise the effective date to require that the revised personal injury protection coverages for basic automobile insurance policies be implemented on the first day of the 18th month after the date of enactment of this bill; and
(5) make certain technical changes.
The bill is now on second reading and ready for a Senate floor vote.
In addition, work on a new PIP Fee Schedule is in progress. The goal is to have it in place by the end of 2025.
NJSIPP Executive Director Andy Kaufman, MD was a featured panelist at BioNJ’s Patient Advocacy Forum on December 13. The topic of the panel was “The Impact of Pharmacy Benefit Management Practices on Patients”
Dr. Kaufman ‘s participation was highly acclaimed and of great assistance to the patient advocates in attendance.
|
|
|
Dr. Woska being honored for his service to our society as the Executive Director for the past 5 years. We congratulate him for all the time and effort that he put forth and the excellent job that he did.
-
Proposed Medicare National Coverage Determination for Epidural Steroid Injection
Effective 12/05/2021
The link to the policy is available here:
https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=38994&ver=7&bc=0
Covered Indications
1. Epidural steroid injection (ESI) will be considered medically reasonable and necessary when the following three (3) requirements are met:
-
History, physical examination, and concordant radiological image-based diagnostic testing and supporting one of the following:
Cervical, Thoracic, or Lumbar radiculopathy, radicular pain and/or neurogenic claudication due to central disc herniation, osteophyte or osteophyte complexes, severe degenerative disc disease, producing foraminal or central spinal stenosis.
OR
Post-laminectomy syndrome
Acute herpes zoster associated pain
AND
Symptoms severe enough to impact quality of life or function. An objective pain scale or functional assessment must be performed prior and repeated following intervention to assess response.
AND
Pain duration of at least 4 weeks, and in the inability to tolerate noninvasive conservative care or failure to respond to 4 weeks of noninvasive conservative care.
*Acute herpes zoster pain refractory to conservative care, 4 week wait is not required
2. ESI must be performed under CT or fluoroscopy image guidance with contrast,unless the patient has a documented contrast allergy or pregnancy where ultrasound guidance without contrast may be considered.
3. Transforaminal EPIDURAL steroid injections (TFESIs) involving a maximum of two (2) levels in one spinal region are considered medically reasonable and necessary. It is important to recognize that most conditions would not ordinarily require ESI at two (2) levels in one spinal region.
4. Caudal EPIDURAL steroid injections (CESIs) and interlaminar EPIDURAL steroid injections (ILESIs) involving a maximum of one level are considered medically reasonable and necessary.
5. It is medically reasonable and necessary to perform TFESIs bilaterally only when clinically indicated.
6. Repeat ESI when the first injection:
At least 50% sustained improvement and/or improvement in function.
If patient fails to respond, repeat ESI after 14 days can be performed, using a different approach, level and/or medication if appropriate, with rationale and medical necessity for second ESI documented in EMR.
7. An initial injection of contrast is required to confirm epidural placement unless the patient has a contraindication to contrast. The subsequent epidural steroid injections should include corticosteroids and may be combined with anesthetics or saline.
8. The ESIs should be performed in conjunction with conservative treatments.
9. Patients should be part of an active rehabilitation program, home exercise program or functional restoration program.
*Note: The scales used to measure of pain and/or disability must be documented in the medical record. Acceptable scales include but are not limited to: verbal rating scales, Numerical Rating Scale (NRS) and Visual Analog Scale (VAS) for pain assessment, and Pain Disability Assessment Scale (PDAS), Oswestry Disability Index (ODI), Oswestry Low Back Pain Disability Questionnaire (OSW), Quebec Back Pain Disability Scale (QUE), Roland Morris Pain Scale, Back Pain Functional Scale (BPFS), and the PROMIS profile domains to assess function.
Limitations
1. Injections performed without image guidance or by ultrasound are not considered reasonable and necessary except in cases of documented contraindication to contrast media (e.g., allergy, pregnancy)
2. ESIs performed with biologicals or other substances not FDA designated for this use is considered investigational and not medically reasonable and necessary
3. It is not considered medically reasonable and necessary to perform multiple blocks (ESI, sympathetic blocks, facet blocks, trigger point injections etc.) during the same session as ESIs, with the exception of a facet synovial cyst and ESI performed in the same session
4. Use of Moderate or Deep Sedation, General Anesthesia, and Monitored Anesthesia Care (MAC) is usually unnecessary or rarely indicated for these procedures and therefore not considered medically reasonable and necessary.Even in patients with a needle phobia and anxiety, typically oral anxiolytics suffice. In exceptional and unique cases, documentation must clearly establish the need for such sedation in the specific patient.
5. ESIs to treat non-specific low back pain (LBP), axial spine pain, complex regional pain syndrome, widespread diffuse pain, pain from neuropathy from other causes, cervicogenic headaches are considered investigational and therefore are not considered medically reasonable and necessary.
6. ESIs are limited to a maximum of four (4) sessions per spinal region in a rolling twelve (12) month period.
7. It is not considered medically reasonable and necessary for more than one spinal region to be injected in the same session.
8. It is not considered medically reasonable and necessary to perform TFESIs at more than two (2) nerve root levels during the same session.
9. It is not considered medically reasonable and necessary to perform CESIs or ILESIs at more than one (1) level during the same session.
10. It is not medically reasonable and necessary to perform CESIs or ILESIs bilaterally.
11. It is not medically reasonable and necessary to prescribe a predetermined series of ESIs.
12. Steroid dosing should be the lowest effective amount, it is recommended not to exceed 80mg of triamcinolone, 12 mg of betamethasone, 15 mg of dexamethasone per session.
13. It generally would not be considered medically reasonable and necessary for treatment with ESI to extend beyond 12 months.Frequent continuation of epidural steroid injections over 12 months may trigger a focused medical review. Use beyond twelve months requires the following:
a. Pain is severe enough to cause a significant degree of functional disability or vocational disability.
b. ESI provides at least 50% sustained improvement of pain and/or 50% objective improvement in function (using same scale as baseline).
c. Rationale for the continuation of ESIs including but not limited to patient is high-risk surgical candidates, the patient does not desire surgery, recurrence of pain in the same location relieved with ESIs for at least three months
d. The primary care provider must be notified regarding continuation of procedures and prolonged repeat steroid use.
14. ESIs should not be performed when contraindicated including but not limited to: suspected or active localized spinal infection, significant systemic infection, compressive lesions of the spinal cord, conus medullaris or cauda equina, suspicion or major risk factors for cancer.
Update on New Medicare Facet Rules
Dr. Steve Aydin created this informative presentation that was shared during the NJSIPP Annual Fall Meeting. We thank him for sharing his slides below.
URGENT - Medicare proposed reduction in sacral and knee RF reimbursement
This topic was discussed the NJSIPP meeting on Wednesday night. It is Urgent you respond - there are only 10 days left!!!
Medicare has proposed to significantly decrease the payment rates for both the physician and the facility setting for the new knee and SIJ radiofrequency ablation CPT codes that are effective January 1, 2020. These new codes will impact all RFA technologies, both cooled and conventional.
We encourage you to:
1. review the proposed rules and provide comments to Medicare. The deadline for comments is September 27, 2019. Links to the proposed rules and information on how to submit comments are noted at the end of this e-mail. It is very important that Medicare receives comments from as many stakeholders as possible before the Final Rules are released later this year.
2. connect with your societies who participated in the new CPT code development process to express your concerns and encourage them to comment on the proposed rules as well.
Here are some important key facts from the proposed rules:
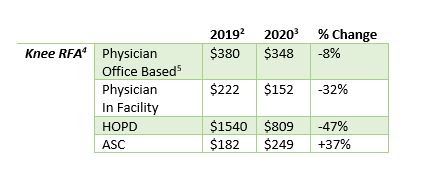
KNEE RFA IMPACTS
- Physician Rule – The proposed physician RVU valuation for the new CPT code for RFA in the knee has significantly decreased from the current peripheral nerve ablation RVUs. Avanos believes the reduction in rate is not appropriate and will likely result in a decrease in usage of this non-opioid alternative to treating pain. Avanos plans to raise this issue in its comment letter.
- Physician & HOPD Rules – The new Knee RFA CPT code was developed to report 3 nerve ablations during a single procedure versus the current peripheral nerve ablation code where reporting is on a per ablated nerve basis.
- HOPD Rule – The hospital outpatient facility payment for the knee RFA procedure has significantly decreased. The new knee RFA CPT code was assigned to the Nerve “Injection” APC (Ambulatory Payment Classification) unlike the new SIJ procedure CPT code which was assigned to the Nerve “Procedure” APC. Avanos believes that the knee RFA procedure should be properly placed in the Nerve Procedure APC and intends to address this in its comment letter.
The following table provides a snapshot of the proposed changes in Medicare National average payment rates:
SIJ RFA IMPACTS
- Physician Rule – The proposed physician RVU valuation for the new code for SIJ RFA procedures has significantly decreased from the current peripheral nerve ablation RVUs. Avanos believes the reduction in rates is not appropriate and will likely result in a decrease in usage of this non-opioid alternative to treating pain. Avanos plans to raise this issue in its comment letter.
- Physician & HOPD Rules – The new SIJ RFA CPT code was developed to report 3 nerve ablations during a single procedure versus the current peripheral nerve ablation code where reporting is on a per ablated nerve basis.
- The following table provides a snapshot of the proposed changes in Medicare National average payment rates.
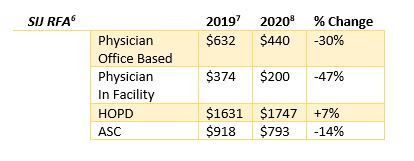
Submit your online comments related to CMS Proposed HOPD:
Submit electronic comments on this HOPD regulation to:
Click on - Regulations.gov – CMS-1717-P
or
https://www.regulations.gov/searchResults?rpp=25&po=0&s=CMS-1717-P&fp=true&ns=true
Follow the instructions under the “Comment Now” tab. Reference Rule CMS-1717-P.
Submit your online comments related to CMS Proposed Physician:
Submit electronic comments on this Physician regulation to:
Click on - Regulation.gov – CMS-1715-P
or
https://www.regulations.gov/searchResults?rpp=25&po=0&s=CMS-1715-P&fp=true&ns=true
Follow the instructions under the “Comment Now” tab. Reference Rule CMS-1715-P.



